|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact Electronic band structureInteraction of atom shellsLike explained at the chapter about atoms, electrons move on fixed orbits around the atomic nucleus. Each of those orbits conforms to a discrete energy level and the electrons are "forbidden" to have other energy levels inside of the atomic shell. Well, that's for true as long as we are looking at a single, insulated atom. We already recognized chemical reactions as a special kind of interaction between the electrons of different atoms. The energy levels of electrons inside of two insulated hydrogen atoms differ significantly from those of two hydrogen atoms being connected to a hydrogen molecule via a covalent bond. Even without forming a chemical bond, the presence of a nearby atom affects the energy levels of the electrons inside of an atomic shell. The discrete levels are shifted, forming orbits with a slightly different energy. If the number of nearby atoms increases, those energy shift of the orbits leads to the formation of a continuous band of energy.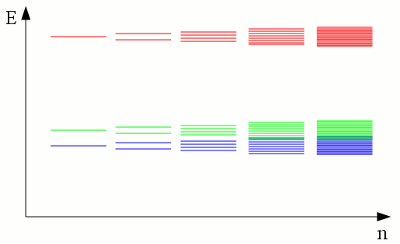
The discrete electron energy of nearby atoms is split into slightly different levels. If a large number of atoms is brought together to form a crystal lattice, a fluid or a gas, the number of orbitals becomes exceedingly large and the difference in energy between the single levels becomes very small. The levels form continuous bands of energy rather than the discrete energy levels of the atoms in isolation. The two lower energy bands (blue and green) overlap, while there is still a gap between the green and the red energy band. ConductivityThe electron shell of an atom is occupied in such a way that the energy level of the whole atom is as low as possible. This condition is called ground state. The outermost electrons of the atoms are called valence electrons. According to this terminology, the outermost energy band is called valence band. By absorbing energy, electrons of an isolated atom can be lifted up to higher orbits or in extreme case they can be separated from it's atomic nucleus (ionization). Those free electrons can be caught by another atom, so that the electron is transferred between two atoms. In a crystal lattice, the energy needed to transfer electrons between two atoms is much lower than the ionization energy, because the atomic nuclei are much closer to each other. The minimum energy needed to transfer electrons between atoms in a crystal lattice can be allocated to an energy band, which is called conduction band: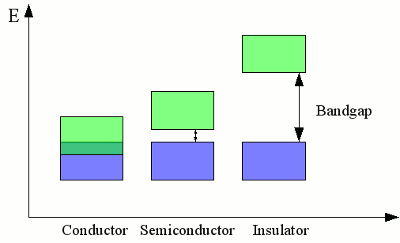
Valence band (blue) and conduction band (green) of different materials. The width and hight of valence band respectively conduction band depend on the used material. If the energy levels of valence band and conduction band overlap, electrons can move between different atoms without additional energy needed. Electrons can move freely within the atomic lattice of those materials, hence an electric current flows as soon as the material is connected to a voltage source. Those materials (usually metals) are called conductors, because they feature a very high conductivity. If conduction band and valence band are separated by a bandgap, electrons can't move freely between different atoms. Energy is needed to lift the valence electrons into the conduction band. Exclusive of the absolute zero point, some electrons are always excited to the conduction band because of the thermal energy. The smaller the forbidden bandgap, the more electrons can be found in the conduction band, hence the conductivity of the material is higher. The higher the bandgap, the fewer electrons are lifted into the conduction band, hence the conductivity is lower. Materials with a small bandgap are called semiconductors, those with a large bandgap are called insulators. The number of electrons being excited to the conduction band is dependent on the environmental temperature, hence the conductivity of semiconductors and insulators increases at higher temperatures. Those materials are called Negative Temperature Coefficient (NTC) thermistors (=resistors). In contrast, the resistance of metallic conductors normally increases with increasing temperature, so those materials are called positive temperature coefficient (PTC) thermistors or posistors. News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|