|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact <<< Electrolysis Galvanic cell >>> Standard electrode potentialNobilityLet's rerun the electrolysis of the previous chapter by using simple table salt (sodium chloride, NaCl) instead of the cupric chloride. Cupric chloride (CuCl2) was turned into the elemental forms of it's ionic components during the process of electrolysis. The deposition of elemental copper could be observed at the cathode and elemental gaseous chlorine was bubbling at the anode. What we expect is to find elemental sodium at the cathode, instead of the copper of the previous experiment and once more gaseous chlorine at the anode. But when running the electrolysis, we will see bubbles coming out of the electrolyte at both electrodes! As expected, chlorine gas is given at the anode. The gas given at the cathode is hydrogen. Why can't we find elemental sodium at the anode? Well, sodium is violently reactive with water and if a small piece of sodium is given in a pot of water, it starts bouncing across the surface of water until it is consumed by the exothermic reaction. The reaction between sodium and water molecules produces sodium hydroxide and hydrogen gas:If we put a piece of copper in a pot of water, nothing happens. When we were talking about the properties of substances at the chapter chemical reaction, one item mentioned was the resistance to corrosion, which is equal to the reactivity with water and oxygen. I guess you already know that platinum, gold and copper are not disintegrated due to chemical reactions with water and oxygen. These metals are called noble metals. On the other hand, base metals like sodium or iron do corrode when exposed to water and oxygen. The nobility of a metal can be discovered by chemical reactions between an elemental metal and the ions of another metal. If we put an iron nail in a solution of cupric chloride, it is quickly changing the color, as metallic copper is deposited at the nail while elemental iron is converted into iron chloride. The chemical equation of this so called single displacement reaction is as follows: When using elemental copper and silver chloride, the copper will be slowly (silver chloride is low soluble in water) covered with elemental silver according to the following reaction: Nothing happens if a piece of copper is put into a solution of iron chloride or silver into cupric chloride. This small reactivity series gives a ranking of the three metals from noble (silver) to reactive (iron) with copper in-between. Dissociation of waterThe chemical elements of the first (main) group of the periodic table tend to form an ionic bond by giving up their single valence electron to an element with high electronegativity. Therefore those elements try to form singly charged cations. The only exception of this rule is hydrogen, because the valence electron of the hydrogen atom is the only electron of the whole atom. By passing it away, the atom would exist without an electron shell! That's why hydrogen doesn't form a crystalline, ionic salt when reacting with an element of the 17th group (halogens), like all other elements of the first group (alkali metals) do. The chemical reaction of hydrogen and chlorine yields hydrogen chloride, which is a colorless gas. One hydrogen atom forms a covalent bond with one chlorine atom resulting in a diatomic molecule.Like explained at the previous chapter, an electric current through an aqueous solution is caused by the flow of ions, hence the conductivity of the electrolyte increases with the number of ions. The conductivity of pure water is very low, but it increases significantly, as soon as some hydrogen chloride is getting in contact with the water. It looks like the hydrogen chloride dissociates, resulting in an aqueous solution of "hydrogen cations" and chloride anions. That's what actually happens - but: The "hydrogen cations" formed in an aqueous solution do not consist of single protons. In a process called protonation of water, hydronium ions (also called oxonium ions) are formed: 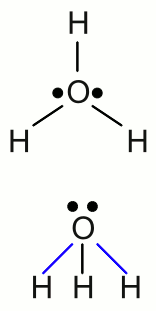 Structure of the hydronium ion:
Structure of the hydronium ion:The singly charged H3O+ cation consists of one oxygen atom forming three covalent bonds to three hydrogen atoms. It has a trigonal pyramid geometry with the oxygen atom at its apex and the angle between the bonds is approximately 113 degrees. Because of the geometry, the ion has a dipole moment with the negative charge localized near the oxygen atom. The upper drawing shows the molecule from the top and the lower drawing from a side view with the blue marked bonds pointing to the front and the black marked bond pointing to the rear. The conductivity of pure water is very low, but different from zero, which indicates the presence of ions. Water molecules can split into hydronium ions and hydroxide ions following the chemical equilibrium: 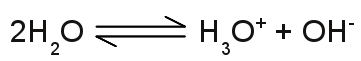
The number of hydronium ions in an aqueous solution is given by p[H], which is the negative logarithm (base 10) of the molar concentration of dissolved hydronium ions (H3O+). A low p[H] indicates a high concentration of hydronium ions, while a high p[H] indicates a low concentration. The definition of pH, which is used nowadays, is slightly different: pH is defined as minus the decimal logarithm of the hydrogen ion activity in a solution. The values of the pH scale reach from 0 to 14 with 7 at the center, which is the pH of pure water, where at the number of hydronium ions equals those of the hydroxide ions. The pH is lower than 7, if there is an excess of hydronium ions (those solutions are said to be acidic) and higher than 7, if there is an excess of hydroxide ions (those solutions are said to be basic or alkaline). An acid is a substance forming hydronium ions when dissolved in water, a base is a substance that forms hydroxide ions when dissolved in water. Standard electrode potentialWhen doing an electrolysis, you have to choose a qualified material used as electrodes. Like explained above, electrodes made of iron are unqualified, especially if cupric chloride is used as electrolyte. The corrosion of the electrodes would start as soon as they get in contact with the aqueous solution. If cupric chloride is the electrolyte, the electrodes should be made of elemental copper or a material with a higher nobility than copper, like silver, gold, platinum or graphite. A very interesting arrangement is using two different materials e. g. copper and iron as a pair of electrodes. You will be able to measure a potential difference between the two electrodes, even if they are not connected to a voltage source! An arrangement like this is some kind of battery. The value of the voltage between two different kinds of electrodes depends generally on the combination of electrode materials, the kind of used electrolyte and the concentration of ions. A very special kind of electrode is the standard hydrogen electrode (SHE). The SHE forms the basis for the comparison with other electrode reactions. The standard hydrogen electrode consists of a platinum electrode dipped in an acidic solution with pure hydrogen bubbled through it. The test arrangement is based on the chemical reaction: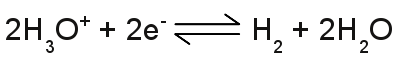
Table of standard electrode potentialThe table below lists the potentials of different metals relative to the standard hydrogen electrode and it is taken from Wikipedia:
RemarksThe nobility of a metal can be determined by the table given above. The less noble metals are listed below the more noble metals.If a metal plate gets in contact with an aqueous solution of ions of a more noble metal, the plate is dissolved while the more noble metal is deposited at the surface of the plate. Hydronium cations can be treated like ions of a metal. If water gets in contact with a less noble, elemental metal, corrosion appears while hydrogen gas is produced. In a mixture of different cations, the metal with the highest (positive) potential gets disposed first. That's why hydrogen gas is bubbling at the cathode while sodium chloride is used as electrolyte in the procedure of electrolysis. <<< Electrolysis Galvanic cell >>> News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|