|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact <<< Primary batteries Heat engines >>> Rechargeable cellsChargingAt the previous chapters we were talking about the chemical processes inside of galvanic cells. Chemical energy is converted into electric energy as soon as a load is connected to the terminals of a primary battery. What about the reverse process - converting electric energy into chemical energy? Well, you can't simply connect a primary battery to a voltage source to initiate the reverse process, but there are special batteries who can be refreshed in that way!As we saw at the previous chapter, zinc is an often used anode material inside of primary batteries. While discharging, the zinc atoms are oxidized to Zn2+ cations, which enter an aqueous solution. The zinc anode functions as the negative terminal of the battery. To initiate the reverse process, the anode has to be connected to the negative terminal of a voltage source and the cathode to the positive terminal. The DC voltage source must provide a higher output voltage than the battery. Otherwise the electrons would flow from the anode of the battery into the negative terminal of the voltage source, meaning it would still be discharged. The reverse process, intended to occur at the zinc electrode is: Zn2+(aq) + 2e- → Zn(s) If we have a look at the Table of standard electrode potential, we can see what actually will happen:2H3O+(aq) + 2e- → H2(g) + 2H2O Remember that the Zn2+ cations are inside of an aqueous solution. Water always dissociates into hydronium and hydroxide ions. During our charging procedure, the zinc electrode operates like the cathode of an electrolytic process. If the hydronium ions get in contact with the zinc electrode, the hydrogen gets reduced to elemental, gaseous hydrogen molecules, prior to the zinc ions, because the standard electrode potential of hydronium ions (0V) is higher than those of Zn2+ (-0.76V). The result is the production of gaseous hydrogen instead of elemental zinc. So never try to recharge primary batteries - they might explode!Like mentioned above, there are special batteries, whose electrochemical reactions are electrically reversible. Such an electrochemical cell is called rechargeable battery, storage battery or secondary cell. To store energy in a secondary cell, it has to be connected to a DC voltage source. The negative terminal of the cell has to be connected to the negative terminal of the voltage source and the positive terminal of the voltage source with the positive terminal of the battery. The voltage output of the source must be higher than those of the cell. By now, the processes running inside of the cell are similar to those described at the chapter electrolysis. Driven by the electric energy of the voltage source, the material at the cathode gets reduced and those at the anode gets oxidized. The material at the cathode is the one featured with the lower standard electrode potential. Inside of the cell, ions are moving between the half cells to balance the charge. 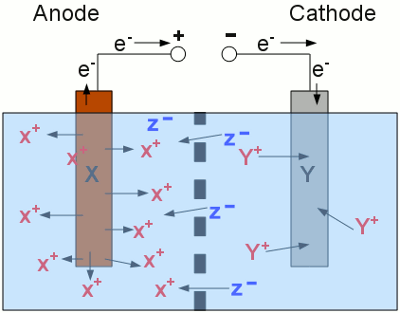
Charging procedure of a secondary cell: The two half cells of the secondary cell are separated by a barrier and the metal electrodes are connected to a DC voltage source. At the anode, metal X gets oxidized (elemental metal atoms X enter the solution as X+ cations) and at the cathode, metal Y gets reduced (Y+ cations are deposited as elemental metal atoms Y). Negatively charged anions are moving from the right to the left side to balance the charge between the two half cells. During the charging procedure, the number of X+ cations is increasing at the left side of the cell and those of the Y+ cations is decreasing at the right side. Metal X is the substance featuring a higher standard electrode potential than metal Y, hence electric energy is consumed during this process and chemical energy is generated. The charging procedure has to be stopped as soon as there are no more cations left around the cathode respectively all of the anode material is transformed into cations. If the cell is still connected to the voltage source, unwanted effects will occur. If the cell contains water (to dissolve the ions), the H2O molecules will be split into gaseous H2 and O2 molecules. This mixture of hydrogen and oxygen is highly explosive! The charging procedures of different rechargeable cells will be discussed at the following chapters of these battery types. DischargingWhile discharging a secondary cell, the electrochemical processes are inverted.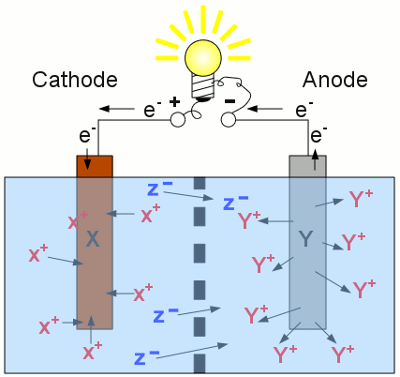
Discharging a secondary cell: As soon as a load is connected to the terminals of a secondary cell, the reverse processes are running inside of each half cell. Now the left half of the cell operates as cathode. The material with the higher standard electrode potential is reduced (the X+ cations are disposed as elemental X atoms) and those with the lower standard electrode potential is oxidized (elemental Y atoms enter the solution as Y+ cations). Compared with the charging procedure, the functionality of anode and cathode are interchanged, and the electrons, as well as the negatively charged anions are running into the opposite direction. The concentration of Y+ cations at the right half is increasing, those of the X+ cations at the left half is decreasing. The discharging procedure stops as soon as there are no more X+ cations left inside of the electrolyte or the whole electrode material Y has entered the solution. To increase the lifetime of a secondary cell, it is beneficial to stop the discharging procedure before the whole electrode material is dissolved! State of chargeAs mentioned at the chapter about batteries, the capacity indicates the amount of electrical energy stored in an electrochemical cell. The State Of Charge (SOC) indicates the percentage of the capacity already/still stored inside the cell during charging/discharging procedure (0-100%). The inverse value is called Depth Of Discharge (DOD).Discharging progressLet's have a look at the voltage output of a battery being connected to a constant load: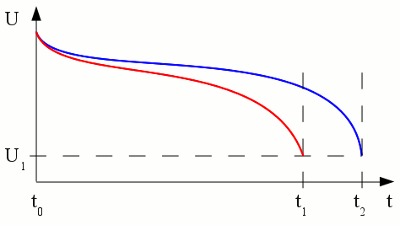
Voltage versus time plotted for the discharge procedure of a battery connected to a constant load. The red graph shows the discharge process while the resistance of the load is lower (higher current). As we can see, the voltage output is not constant over time. The reason for this is the increasing internal resistance (see chapter batteries for details) of the cell, caused by a decreasing concentration of cations around the cathode. The internal resistance of the cell causes a higher voltage drop if a higher load is connected to the terminals of the battery. Depending on the type of battery there is a typical voltage witch should never be underrun during the discharge process. This predetermined terminal voltage is reached at t1 respectively t2 and so the discharge was stopped. Discharging a battery below this voltage causes irreversible damage of the cell! Capacity and loadLike explained at the chapter batteries, the capacity of a cell is a fixed value depending on the amount of chemical substances stored inside of it. The voltage drop, caused by the internal resistance, depends on the magnitude of the current. So if a battery is discharged at a relatively high rate, the available capacity will be lower than expected, because the minimum voltage of the cell is reached more early in time. The capacity given by the manufacturers is usually the product of 20 hours multiplied by the maximum constant current that a new battery can supply for 20 hours at 20 C°. If the capacity printed on a battery is 5Ah it doesn't mean that the battery can supply a current of 5A for one hour. It means that is can supply a current of 0.25A for a period of 20 hourst - time (in hours) that the battery can sustain, QP - capacity when discharged at a rate of 1A, I - discharge current, k - constant (approximately 1.3) So the capacity of a 20Ah lead-acid battery decreases to 10.02Ah if discharged with 10A and to poorly 8.14Ah if discharged with 20A! Another parameter which influences the capacity is the environmental temperature. Low temperature leads to a lower rate of diffusion which increases the internal resistance and so decreases the capacity, too. Self-dischargePrimary as well as secondary cells are loosing charge even if no load is applied to them. This self discharge rate is caused by chemical reactions occurring inside of the cells. Primary batteries can loose 8 to 20% of their initial charge per year.LifespanOn each charge/discharge cycle of a secondary battery, some deterioration occurs. Even if not in use, the electrolytes of the half cells migrate from one to the other half, because the barrier between the two half cells is never as selective as it should be in theory. Furthermore the electrode material is weakened with every cycle and some of the active material may fall off. Another side effect is the formation of crystals inside of the electrolyte or on the surface of the electrodes. The separator material can be penetrated by the formation of crystals which causes shortcuts. All of these effects will gradually decrease the capacity of the cells.Cell balancingA battery often consists of more than one electrochemical cell connected in series. The resulting voltage output is the sum of the voltages generated by the single cells.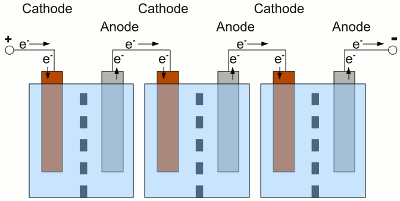
Electrochemical cells connected in series. The used cells should be of the same type, have the same capacity and of course the same state of charge. What happens if one of the cells is completely discharged, while the state of charge of the other cells is still high? The cells and the load are connected in series, hence the same current is running through all devices: 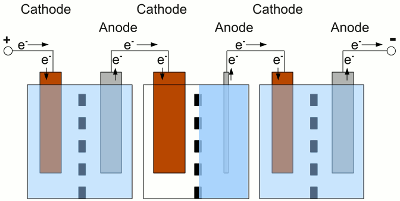
The middle cell of the battery is completely discharged, the neighbor cells still provide electric energy. Because of the fact that the neighbor cells still provide electric energy, electrons are injected into the cathode of the discharged cell, but there are no more cations which could be reduced to elemental atoms. With water used as solvent, the now occurring electrolysis process forms elemental, gaseous hydrogen. At the anode of the discharged cell, electrons are sucked off and more electrode material than usual is entering the solution. Caused by this, the electrode can crack. This process is called reverse charging, because it is similar to a charging procedure with the battery connected to a charging circuit the wrong way round. Even if the middle cell is not completely discharged, but the current running through the whole battery is high enough, the internal resistance of the middle cell can experience a reverse voltage that is higher than the remaining internal forward voltage. The polarity of the middle cell is reversed while the current flows through the battery. Depending on the type of battery and used solute, many side reactions will occur, which shorten the lifespan of the battery. To avoid a deep discharge of a single cell, the cells of a battery have to be balanced during charging procedure to guarantee that all of the cells of one battery have the same state of charge. Tracing the output voltage of all cells inside of a battery during the discharging procedure makes it possible to detect a weak cell before it will be destroyed. The state of charge can be detected via the output voltage of a cell or the complete battery when considering the current running through the device. The discharging process must stop as soon as the lowest voltage of the cell or battery is reached. The electronics or software used to observe the output voltages, is called battery management. Observing single cells is very rare, because of the complexity of the required hardware (cabling). Usually the output voltage of the whole battery, thus the voltage of multiple cells is observed by the electronic circuits. The handicap of observing complete batteries is the fact that one weak cell inside of this battery is hard to detected. EfficiencyCaused by the internal resistance of a battery, there are energy losses while charging/discharging a storage battery. So besides the losses of the battery charger, the control electronic of our project car and the efficiency of the electric motor, we must also consider the losses during the charge and discharge process of the battery. Depending on the current running through the devices, these losses are significant!Data about the efficiency of different types of rechargeable batteries will be presented at the following chapters. <<< Primary batteries Heat engines >>> News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|