|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact <<< Galvanic cell Corrosions prevention >>> CorrosionRustAs we could see at the table about the standard electrode potential, iron is less noble than hydrogen, hence if elemental iron gets in contact with hydronium ions, the metal gets dissolved. Hydronium ions are formed by the dissociation of water, creating hydronium ions and hydroxide ions, like explained at the chapter about the standard electrode potential. The hydronium ions trigger the chemical reaction with iron:This kind of corrosion appears very slowly in pure water, because of the low number of hydronium ions. The hydroxide ion, created by the dissociation of the water molecule is not listed at the chemical equation above, but the product formed is iron hydroxide. So a side effect of the reaction is an alkaline solution, which decreases the number of hydronium ions. If the water becomes acidic, the rate of corrosion increases significantly. Another form of corrosion is the chemical reaction with oxygen and water: Once more, iron(II) hydroxide is created by the reaction above, but without hydronium ions needed. Iron(II) hydroxide is not the final product, because at the presence of water and oxygen, the following chemical reaction occurs: Iron(III) hydroxide is still not the final stage of the corrosion process: The substance created by the chemical reaction above is called Iron(III) oxide-hydroxide in a hydrated form, which is expressed by the addition "* H2O". The water molecules are an integral part of the crystal and not separated from the ionic compound which would be written as "+ H2O". Iron(III) oxide-hydroxide is poorly soluble in water and it can react to the product iron(III) oxide: The water molecules formed during this chemical reaction are not enclosed by the crystal lattice of the iron(III) oxide. The Iron(II) hydroxide can also react to iron(II) oxide instead of iron(III) hydroxide: Rust is a series of iron oxides formed by the reaction of iron and oxygen in the presence of water. Galvanic corrosionA process similar to those inside of a galvanic cell occurs whenever different metals electrically contact each other and are dipped into an electrolyte. This process is called galvanic corrosion. Let's have a look at an arrangement of iron and copper: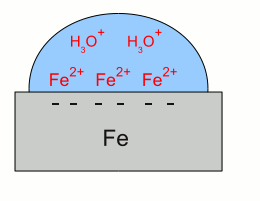 Galvanic corrosion:
Galvanic corrosion:Iron gets in contact with water. Like described at the chapter about galvanic cells, iron atoms enter the water as iron cations, leaving their electrons inside of the iron "electrode". The electrons and the iron ions are attracted by coulomb forces, resulting in a layer of iron ions at the surface of the metallic iron. Those layer of iron ions prevents hydronium ions from getting in contact with the metal to get the super-numerous electrons. Otherwise the redox reaction between hydronium ions and iron would start the formation of elemental hydrogen and so the corrosion of the iron as described above. 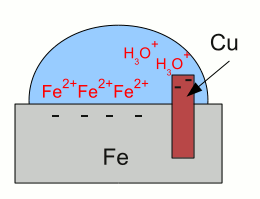 The situation alters as soon as the iron gets in contact with copper. The super-numerous electrons move into the copper "electrode" like they do in a galvanic cell. The hydronium ions can get in contact with the metal surface of the copper, hence with the super-numerous electrons. The reduction of hydronium ions to elemental hydrogen molecules decreases the number of electrons, hence more iron atoms can enter the solution leaving their electrons inside of the metal.
The situation alters as soon as the iron gets in contact with copper. The super-numerous electrons move into the copper "electrode" like they do in a galvanic cell. The hydronium ions can get in contact with the metal surface of the copper, hence with the super-numerous electrons. The reduction of hydronium ions to elemental hydrogen molecules decreases the number of electrons, hence more iron atoms can enter the solution leaving their electrons inside of the metal.
Galvanic corrosion effects a corrosion of the less noble metal at an accelerated rate. <<< Galvanic cell Corrosions prevention >>> News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|