|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact Corrosion preventionBuilt-in corrosion preventionBase metals like aluminum, zinc and titanium are less noble than hydrogen or even iron, but nevertheless they have a high resistance to weathering, particularly to corrosion - so why?Well, those metals are indeed very reactive with atmospheric oxygen and for this reason they form metal oxides on any exposed surface. However the oxidation process is limited to a very thin layer, because the metal oxides, formed by the chemical reaction, establish a coating, which prevents further corrosion processes by locking out the oxygen molecules from the metal. The layer of metal oxide is insoluble in water and strongly bound to the metal beneath. This hard, non-reactive surface film, inhibiting further corrosion is called passivation layer. In an electrolytic process called anodizing, the thickness of the natural oxide layer can be increased. But why doesn't iron oxide prevent steel from further corrosion? Because the oxides formed in the chemical reactions with water and oxygen are just weakly bound to the iron beneath. As seen at the previous chapter, rust is a mixture of different iron oxides. Rust is more bulky than iron, so these compounds flake off from the surface, exposing the fresh iron beneath and so leading to a continuous corrosion process. Stainless steelSteel alloys with a content of more than 11% chromium by mass are called stainless steel, inox steel or corrosion-resistant steel (CRES). The amount of chromium in stainless steel alloys is sufficient to form a passive film of chromium(III) oxide (Cr2O3). In contrast to the iron oxide layer of carbon steel, which is just loosely bound to the bulk steel, the chromium oxide forms a hard and compact film being strongly bound to the steel beneath it. When this protective layer is scratched, it reforms quickly by the formation of new chromium oxide. Stainless steel is still lustrous, because the layer of chromium(III) oxide is too thin to be visible.Only a few cars, like the De Lorean DMC-12 (you might know it from "Back to the future") are made of stainless steel. Unfortunately, the project car isn't one of them... If stainless steel gets in contact with common (carbon) steel, the carbon steel corrodes in an accelerated rate, because of galvanic corrosion. So it isn't a good idea to weld stainless steel on carbon steel to repair a rusted bodywork. Sacrificial anodeUntil now, we recognized galvanic corrosion as a negative effect, but it can also be used to protect a metal from corroding. If iron is the metal intended to protect, it has to be electrically connected to a less noble metal like zinc: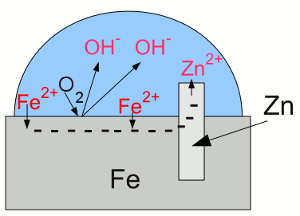 "Galvanic cell" with iron and zinc:
"Galvanic cell" with iron and zinc:Zinc is less noble (= more active) than iron, thus more of the zinc atoms than of the iron atoms are loosing their electrons and enter the electrolyte. The electrons move from the zinc to the iron "electrode", boosting the process of reduction at the surface of the iron. Hence iron atoms who entered the solution as cations are reduced and disposed as iron atoms at the surface. Essential is the fact, that the oxygen molecules being reduced to hydroxide anions are gaining the needed electrons from the zinc. Without the zinc "electrode", the electrons needed for the reduction process of the oxygen molecules would be furnished by the iron which would become oxidized. At the given arrangement, the zinc "electrode" operates as anode, because it releases positively charged cations and attracts negatively charged anions. Iron acts as cathode by attracting cations and boosting reduction processes. The corrosion process of the zinc anode protects the iron cathode from becoming oxidized, so the term "sacrificial anode" is used. Attaching a piece of zinc electrically conductive at the bodywork won't prevent your car from rusting, because to make the sacrificial anode work, the zinc anode and the whole iron cathode has to be covered with an electrolyte, too. You would have to put a piece of zinc into each water drop at the bodywork of your car, which is hard to realize. Applied coatingsCoating iron or steel with zinc is a very common way to protect iron from corrosion. The zinc layer itself is protected by passivation as described above and the underlaying steel is insulated from oxygen and water. Using zinc has another advantage: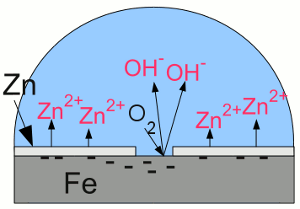 Scratched zinc coating:
Scratched zinc coating:If the zinc coating gets scratched, the zinc layer acts like a sacrificial anode, hence the exposed iron works as a cathode, boosting reduction processes. The deposition of zinc or other metallic coatings at the surface of another metal is called galvanization. Hot-dip galvanizing is a process of coating a metal with a zinc layer by passing it through a bath of molten zinc. The metal is heated up to temperatures of about 460°C, which can provoke distortion of the structure. The advantage of hot-dip galvanization is a metallurgical bond between zinc and steel. electrogalvanization or electroplating is a process using electrical current to coat conductive objects with a thin layer of zinc or other metals. It is an electrolytic process using the object to be plated as the cathode while the anode is made of the material to be platened at the object. Using zinc paint is another way to cover steel with zinc: 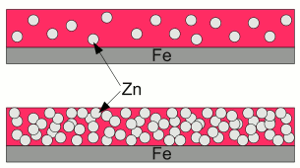 Zinc paint:
Zinc paint:At the upper part of the drawing, the number of zinc grains is too low. The particles are insulated from each other and from the surface of the iron. There is no electrically conductive connection, hence the zinc can't operate as sacrificial anode. The "zinc coating " is nothing but (expensive) grey painting. At the lower part of the drawing, the zinc grains can operate as sacrificial anode, because they touch each other and the surface of the iron. The disadvantage is, that there is not much binder left, which leads to a poor adhesion between the zinc grains and the iron beneath. To my mind, zinc spray is more often than not, just a Grey colored paint of poor quality. A well done painting forms a very adhesive coating, which isolates the iron effectively from the corrosive environment. A very cost-effective substance, preventing iron from corrosion, is grease or wax. A coating with these substances is water-repellent and very flexible. Enclosed box sections of cars are often treated by injections of so called slushing oil. Wherever I found oil residues, I could not find iron oxide - that's the conclusion of the disassembling procedure of my two (very rusty) 2CVs. Rust convertersSubstances converting iron(III) oxide (Fe2O3) into iron(II, III) oxide (Fe3O4) or ferric phosphate (FePO4) are often called rust converters. A very common chemical product called rust converter, is phosphoric acid:After treatment, the black ferric phosphate or magnetite (Fe3O4) coating can be scrubbed off, leaving the blank metal surface (which can be done without this treatment, too). If left in place it acts like a (very poor) rust protection. The adhesion of the converted iron oxide layer is weak. Rust is not reconverted into iron, but in another form of iron oxide. The only way to reconvert rust into brand new steel is using a flex and a welding machine! Use special grease instead of miracle cures to stop corrosion wherever rust must be left in place (enclosed box sections)! News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|