|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact <<< Model motor Chemical reaction >>> AtomsAtomic theoryAtoms are the base unit of the matter surrounding us. The name atom comes from the Greek word for "indivisible". At the end of the 19th and the beginning of the 20th century it was discovered that atoms are nohow indivisible. 1897 while working with cathode rays Joseph John Thomson found out that negative charged electrons are a component of atoms. He postulated that atoms consist of a uniform sea of positive charge with the negative charged electrons distributed through. The charge of the positive sea balances those of the electrons.Well known is the Rutherford scattering done by Ernest Rutherford in 1909. He used a very thin gold foil being shot by alpha particles (twice positive charged helium nuclei) and found out that the positive charge and almost all of the mass of atoms are concentrated on a small area. This led to the development of the planetary model of the atom, meaning the electrons are moving around the atomic nucleus like planets around the sun shielding the positive charge. In 1913 the Danish physicist Niels Bohr published his concept of atoms. He suggested that electrons are confined to clearly defined orbits around the atomic nucleus. According to his model electrons can't move in intermediate states between those orbits. The electrons "jump" between the orbits by absorbing or emitting a specific amount of energy (=quantum) given by electromagnetic radiation (=light). With the help of these orbital transitions the appearance of fixed lines in a spectrum could be explained successfully. With the help of the concept of wave-particle duality (Louis de Broglie, 1924), the Schrödinger equation (Erwin Schrödinger, 1926) and the uncertainty principle (Werner Heisenberg, 1926) the concept of atomic orbitals was developed. The mathematical function of this atomic model calculates the probability of finding any electron of an atom in any specific region around the atom's nucleus. The last "why?" and "how?" concerning the movement of electrons around the atomic nucleus is still open and an object of research. To understand chemical reactions it is important to know that an atom consist of negative charged electrons moving in quantized orbits around the positive charged atomic nucleus. 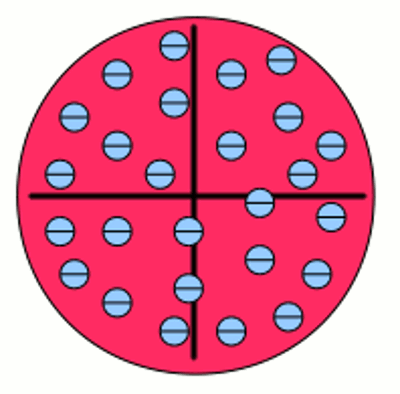
Iron atom according to Thomson: The negative charged electrons (blue) are embedded to a sea of positive charge (red), similar to a plum pudding. That's why this concept of atoms is known as the plum pudding model. The chemical properties of atoms are given by the number of protons respectively electrons. Inside of a uncharged, neutral atom the number of electrons in the electron shell equals those of the protons of the atomic nucleus. The charge of a proton is 1,602 176 487(40) * 10-19 C, those of an electron -1,602 176 487(40) * 10-19 C. Because of the high difference of mass between protons (1,672 621 637(83) * 10-27 kg) and electrons (9,109 382 15(45) * 10-31 kg) the movement of the atomic nucleus can be neglected. Neutrons ar one more component of atomic nuclei. Those (outwards) uncharged particles with a mass of 1,674 927 211(84) * 10-27 kg increase the mass of the atomic nucleus. In the majority of cases the number of neutrons equals those of the protons in an atomic nucleus. Variations in the number of neutrons (while the number of protons is constant) in an atomic nucleus are called isotopes. Because of the fact that neutrons don't affect chemical reactions, they will be neglected in the following. Electron shellAs mentioned above electrons are moving on specific orbits (also called shells) around the atomic nucleus. Each orbit conforms to an energy level and the arrangement of the electrons results in a total energy of the electron shell. The electrons are attracted by the positive charged nucleus. Based on the Bohr model which is used because of it's demonstrative character, there is a minimum distance between electrons and nucleus which can't be under-run. Why? Well there is still no fundamental answer to that question. Besides the attractive force between electron and nucleus there are repellent forces acting between the electrons. Hence not all electrons are arranged in the shell with the minimum distance possible. According to the "Aufbau principle" (German word for building up), each shell consists out of one or more subshells. The shells are labeled with characters starting with K, L, M, N.. or by numbers (1, 2, 3, 4, ...). The subshells are labeled by small characters (s, p, d, f, g, h) and each of them can contain only a fixed number of electrons. The maximal number of electrons in a subshell is as follows: s=2, p=6, d=10, f=14, g=18... .The number of subshells is given by: K=1, L=2, M=3, N=4..., whereby the maximum number of electrons in a shell is given by:K=2, L=8, M=18, N=32... . The shells and subshells are not filled in ascending sequence but (mostly) following the given scheme: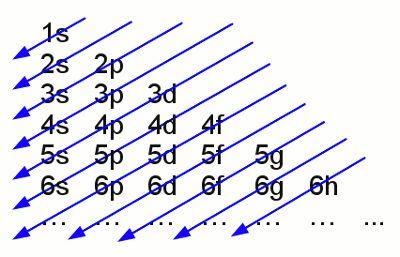
Electron configuration of the shells No rule without exception - slight variations are given amongst others at the atoms copper, chromium , silver, platinum and gold. 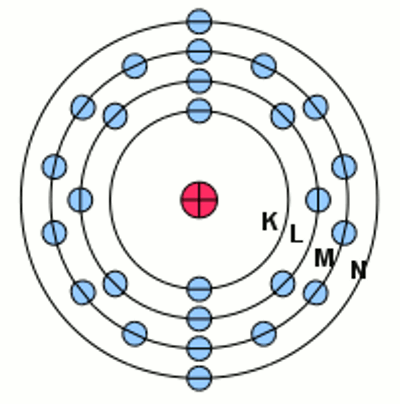
Iron atom at it's ground state according to the shell model: The negative charged electrons (blue) are moving at circular orbits (shells) around the positive charged nucleus considered to be stationary. The electron shell of an atom is described by the filled subshells. The labeling is given by the number of the shell, the character of the subshell and the number of electrons inside the subshell as a superscript number. For iron we get the notation: The outermost electrons of an atom are called valence electrons. IonizationLike mentioned at the Bohr model, electrons are moving on fixed orbits around the atomic nucleus. The state with the lowest energy possible is called the ground state. The elecron configuration scheme given above leads to those ground state of the shells. By absorbing energy, electrons can move to higher subshells as long as those subshell is not filled with the maximum number of electrons. If one ore more electrons are in a subshell of a higher energy level, the total energy of the electron shell is higher than the ground state. This state is called excited state. The electrons inside an atom absorb energy in certain portions (quanta) while jumping between different subshells. From the excited state they fall back to their ground state after a small span of time by emitting electromagnetic radiation (light). At the chapter about electric charge we have learned that a certain amount of energy is released if two different charged particles are approaching from infinite to a given distance. Vice versa this energy is needed to move the particles from the given distance to the infinite. In a similar way the electrons inside an atom can be moved to a shell with an infinite diameter so that the electron is separated from the now positively charged restatom. This procedure is called ionization, the remaining positively charged atom is called ion, the required amount of energy is called ionization potential. If there is more than just one electron inside of the atom, they can also be separated from the atomic nucleus. The ionization potential of the second, third or fourth electron is always higher than those of the first one, because the atom is already a one, two or three times positively charged ion.<<< Model motor Chemical reaction >>> News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|